Pigments and absorption spectra
A chromophore is the moiety within the pigment molecule that is responsible for the molecule's color. The chromophore possesses two orbitals whose difference in energy falls within the light spectrum. Thus, a photon of incident light is able to excite an electron from its ground-state orbital to the excited state.
Chromophores typically exist as conjugated pi systems or metal complexes:
1. In conjugated pi systems, electron excitation occurs between pi orbitals spread across alternating single and double bonds. Examples of conjugate pi chromophores are retinenes, azo compounds, lycopene, β-carotene, and anthocyanins.
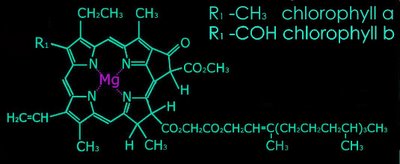
2. Metal complex chromophores possess share d-orbitals between transition metals (with incomplete d-shells) and ligands. Examples of such chromophores are the chlorophylls, hemoglobin, and hemocyanin.
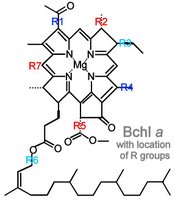
Commonly, chromophores comprise four pyrrole rings;
a. open chain pyrroles without a metal atom: phytochromes and phycobilins, carotenoids
b. pyrroles arranged as a porphyrin ring with a central metal atom: chlorophylls, bacteriochlorophylls
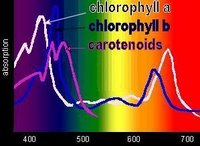 Chlorophyll absorbs all wavelengths of visible light except green, which it reflects, producing the green color of leaves. Various chlorophylls and accessory pigments have characteristic absorption spectra. The action spectrum of photosynthesis relates to the relative electron-exciting effectiveness of different wavelengths of light.
Chlorophyll absorbs all wavelengths of visible light except green, which it reflects, producing the green color of leaves. Various chlorophylls and accessory pigments have characteristic absorption spectra. The action spectrum of photosynthesis relates to the relative electron-exciting effectiveness of different wavelengths of light.When a pigment absorbs light energy, either:
a. energy is dissipated as heat, or
b. energy is re-emitted immediately at a longer wavelength (fluorescence), or
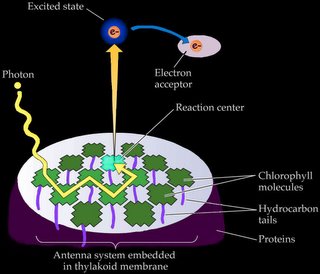
c. "exciton" energy is passed from one molecule of chlorophyll to another in the photosynthetic antenna, and
d. an energetic electron is ejected by a pair of chlorophylls at the reaction center, initiating the electron transport chain that generates concentration gradients and the energy-storage molecules employed by the Calvin cycle to generate glucose and starch.
Chlorophyll triggers the photosynthetic chemical reactions of 'c' and 'd' only when it is associated with proteins embedded in a membrane (as in a chloroplast) or with the membranous infoldings of photosynthetic prokaryotes such as Cyanobacteria and Prochlorobacteria.
Accessory pigments include chlorophyll b and chlorophylls c, d, and e in algae and protistans, plus xanthophylls, and carotenoids. Non-chlorophyll accessory pigments absorb light energy at wavelength that do not stimulate chlorophyll. The energy absorbed by accessory pigments is funnelled to the reaction center for conversion into chemical energy.
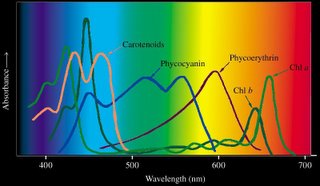 Beta-carotene is a yellow carotenoid pigment. Phycobilins are water-soluble pigments, so they are found in the cytoplasm, or in the stroma of the chloroplast. Phycolibins occur in Cyanobacteria (phycocyanin and phycoerythrin) and the "red algae", the Rhodophyta (phycoerythrin). Bacteriochlorophylls (Bchl) is slightly different chemically from plant chlorophyll – one of the porphyrin rings is saturated – and it absorbs light of longer wavelength (lower energy) than chlorophylls. The bacteriochlorophyll b of Rhodopseudomonas viridis absorbs light of wavelength 960 nm.
Beta-carotene is a yellow carotenoid pigment. Phycobilins are water-soluble pigments, so they are found in the cytoplasm, or in the stroma of the chloroplast. Phycolibins occur in Cyanobacteria (phycocyanin and phycoerythrin) and the "red algae", the Rhodophyta (phycoerythrin). Bacteriochlorophylls (Bchl) is slightly different chemically from plant chlorophyll – one of the porphyrin rings is saturated – and it absorbs light of longer wavelength (lower energy) than chlorophylls. The bacteriochlorophyll b of Rhodopseudomonas viridis absorbs light of wavelength 960 nm.The ability to absorb some energy from the longer, more penetrating wavelengths probably confered an advantage to early photosynthetic algae below the upper zone of the sea (photic or euphotic or epipelagic). Depending upon clarity of water, the shorter, high energy wavelengths penetrate very little below 5 meters in sea water (euphotic zone).
HOME • • Section Photosynthesis • Calvin cycle • C-3 • C-4 • CAM • Chloroplast • Chlorosomes • Cyanobacterial cell : cyclic photophosphorylation : • Light-reactions : noncyclic photophosphorylation : • Nonoxygenic photosynthesis • Oxygenic photosynthesis • Photosynthesis Overview • Photophosphorylation • Plant cell • Timeline • • Section Pigments • Antenna and Reaction Center • Bacteriochlorophylls • Carotenoids • Chlorophylls and accessory pigments • Pigments and absorption spectra • Phycobilins Section Articles • SITE MAP •
External link External link Pigments and absorption spectra